碳酸氢钠

产品属性
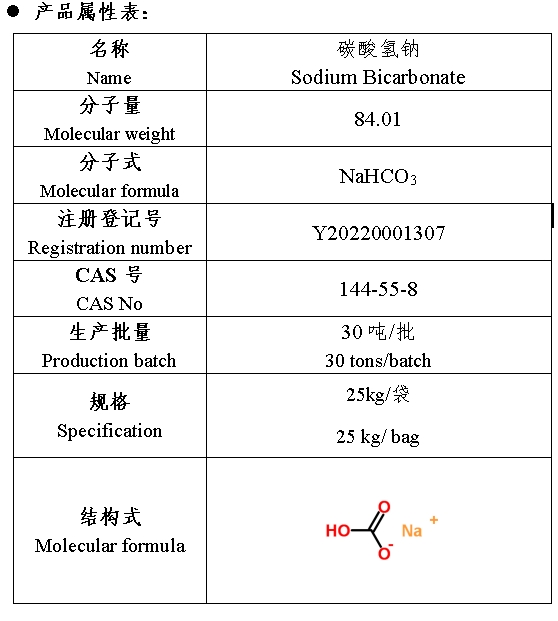
名称:碳酸氢钠
外观性状:白色粉末
级别:医药级
CAS号:144-55-8
分子式:NaHCO3
规格:25kg
产品介绍
l 碳酸氢钠产品介绍
碳酸氢钠为抗酸药,用于治疗代谢性酸中毒、碱化尿液等。也可作为制酸药,治疗胃酸过多引起的症状。
Sodium bicarbonate is used as antacid for the treatment of metabolic acidosis and alkalization of urine. It can also be used as antacid to treat symptoms caused by excessive gastric acid.
l 产品优势 The advantages of product
品质优良 Excellent quality
质量标准严,杂质限度低。颗粒饱满光滑,粒度分布均匀。
Strict quality standard and low impurity limit. The particles are full and smooth, and the particle size distribution is uniform.
设备先进 Advanced equipment
采用新型专利设备,实现全自动化生产运行,效率高、产量稳定、产品颗粒分明。
With the adoption of new patented equipment, fully automated production and operation are realized, with high efficiency, stable output and distinct product particles.
品类齐全 Complete categories
攻克大颗粒碳酸氢钠技术壁垒,原料药、试剂、药用辅料三个级别均可供应,适用于口服、注射、血液透析等多种范围。
Overcoming the technical barrier of large granular sodium bicarbonate, API, reagents and pharmaceutical excipients can be supplied at three levels, which are suitable for oral administration, injection, hemodialysis and other fields.
l 质量标准对比
项目 Items | 欧洲药典 EP | 企业质量标准 Enterprise quality standard |
外观 Appearance | 本品为白色或类白色结晶性粉末,在干燥状态或在溶液中加热时,逐渐变成碳酸氢钠 This product is white or white-like crystalline powder, which gradually becomes sodium bicarbonate when it is dry or heated in solution. |
本品为白色结晶性粉末;无臭;在潮湿空气中即缓缓分解;水溶液放置稍久,或振摇,或加热,碱性即增强。 This product is white crystalline powder; Odorless; Decompose slowly in damp air; The alkalinity of the aqueous solution increased when it was left standing for a little longer, either by shaking or by heating. |
溶解度 Solubility | 本品在水中溶解,在乙醇(96%)中几乎不溶 It is soluble in water, practically insoluble in ethanol (96%) | 本品在水中溶解,在乙醇中不溶 It is soluble in water, insoluble in ethanol |
钠盐 Sodium salt | 应符合规定 Comply with the regulation | 应符合规定 Comply with the regulation |
碳酸氢盐 Bicarbonate | 应呈正反应 Positive reaction | 应呈正反应 Positive reaction |
碱度 Basicity | pH≤8.6 | pH≤8.6 |
溶液澄清度 Clarity of solution | 应澄清无色 Should be clear and colorless | 应澄清(供注射、血液透析用)或不得更浓于2号浊度标准液(供口服用) Should be clear (for injection, hemodialysis) or no thicker than No.2 turbidity standard solution (for oral use) |
氯化物 Chloridate | 不得更浓(0.015%) Not thicker (0.015%) | 不得更浓[(0.002% (供注射、血液透析用)或0.015% (供口服用)] Not thicker [(0.002% (for injection, hemodialysis) or 0.015% (for oral use)] |
硫酸盐 Sulphate | 不得更浓(0.015%) Not thicker (0.015%) | 不得更浓[(0.005% (供注射、血液透析用)或0.015% (供口服用)] Not thicker [(0.005% (for injection, hemodialysis) or 0.015% (for oral use)] |
铵盐 Ammonium salt | 不得更深(0.002%) No deeper (0.002%) | 不得更深(0.002%) No deeper (0.002%) |
干燥失重 Loss on drying | ≤0.25% | ≤0.23% |
元素杂质 Elemental impurity | —— | 铝≤0.0002%; 铜≤0.0001%; 镁≤0.0002% Al≤0.0002%; Cu≤0.0001%; Mg≤0.0002% |
钙盐 Calcium salt | ≤0.01% | 不得更浓[(0.01%(供注射、血液透析用)] Not thicker [(0.01% (for injection, hemodialysis)] |
铁盐 Molysite | ≤0.002% | 不得更浓[(0.0005%(供注射、血液透析用)或0.0015(供口服用)] Not thicker [(0.0005% (for injection, hemodialysis) or 0.0015% (for oral use)] |
重金属 Heavy metal | —— | ≤5PPM |
砷盐 Arsenic salt | —— | 应符合规定 Comply with the regulation |
细菌内毒素 Bacterial endotoxin | —— | <20EU/g |
微生物限度 Microbial limit | —— | 需氧菌总数不得过800cfu/g,霉菌及酵母菌数不得过80cfu/g,大肠埃希菌不得检出(口服用);需氧菌总数不得过80cfu/g,霉菌和酵母菌总数不得过10cfu/g,大肠埃希菌不得检出(注射用) Total aerobic microbial count ≤800cfu/g, Total yeasts and molds ≤80cfu/g, Escherichia coli should not be detected (for oral use) or Total aerobic microbial count ≤80cfu/g, Total yeasts and molds ≤10cfu/g, Escherichia coli should not be detected (for injection) |
含量 Content | 含NaHCO3应为99.0~101.0% NaHCO3 content should be 99.0~101.0% | 含NaHCO3应为99.5~100.5%(供注射、血液透析用)或不得少于99.0%(供口服用) NaHCO3 content should be 99.5~100.5% (for injection, hemodialysis) or no more than 99.0% (for oral use) |
项目 Items | 欧洲药典 EP | 企业质量标准 Enterprise quality standard |
外观 Appearance | 本品为白色或类白色结晶性粉末,在干燥状态或在溶液中加热时,逐渐变成碳酸氢钠 This product is white or white-like crystalline powder, which gradually becomes sodium bicarbonate when it is dry or heated in solution. |
本品为白色结晶性粉末;无臭;在潮湿空气中即缓缓分解;水溶液放置稍久,或振摇,或加热,碱性即增强。 This product is white crystalline powder; Odorless; Decompose slowly in damp air; The alkalinity of the aqueous solution increased when it was left standing for a little longer, either by shaking or by heating. |
溶解度 Solubility | 本品在水中溶解,在乙醇(96%)中几乎不溶 It is soluble in water, practically insoluble in ethanol (96%) | 本品在水中溶解,在乙醇中不溶 It is soluble in water, insoluble in ethanol |
钠盐 Sodium salt | 应符合规定 Comply with the regulation | 应符合规定 Comply with the regulation |
碳酸氢盐 Bicarbonate | 应呈正反应 Positive reaction | 应呈正反应 Positive reaction |
碱度 Basicity | pH≤8.6 | pH≤8.6 |
溶液澄清度 Clarity of solution | 应澄清无色 Should be clear and colorless | 应澄清(供注射、血液透析用)或不得更浓于2号浊度标准液(供口服用) Should be clear (for injection, hemodialysis) or no thicker than No.2 turbidity standard solution (for oral use) |
氯化物 Chloridate | 不得更浓(0.015%) Not thicker (0.015%) | 不得更浓[(0.002% (供注射、血液透析用)或0.015% (供口服用)] Not thicker [(0.002% (for injection, hemodialysis) or 0.015% (for oral use)] |
硫酸盐 Sulphate | 不得更浓(0.015%) Not thicker (0.015%) | 不得更浓[(0.005% (供注射、血液透析用)或0.015% (供口服用)] Not thicker [(0.005% (for injection, hemodialysis) or 0.015% (for oral use)] |
铵盐 Ammonium salt | 不得更深(0.002%) No deeper (0.002%) | 不得更深(0.002%) No deeper (0.002%) |
干燥失重 Loss on drying | ≤0.25% | ≤0.23% |
元素杂质 Elemental impurity | —— | 铝≤0.0002%; 铜≤0.0001%; 镁≤0.0002% Al≤0.0002%; Cu≤0.0001%; Mg≤0.0002% |
钙盐 Calcium salt | ≤0.01% | 不得更浓[(0.01%(供注射、血液透析用)] Not thicker [(0.01% (for injection, hemodialysis)] |
铁盐 Molysite | ≤0.002% | 不得更浓[(0.0005%(供注射、血液透析用)或0.0015(供口服用)] Not thicker [(0.0005% (for injection, hemodialysis) or 0.0015% (for oral use)] |
重金属 Heavy metal | —— | ≤5PPM |
砷盐 Arsenic salt | —— | 应符合规定 Comply with the regulation |
细菌内毒素 Bacterial endotoxin | —— | <20EU/g |
微生物限度 Microbial limit | —— | 需氧菌总数不得过800cfu/g,霉菌及酵母菌数不得过80cfu/g,大肠埃希菌不得检出(口服用);需氧菌总数不得过80cfu/g,霉菌和酵母菌总数不得过10cfu/g,大肠埃希菌不得检出(注射用) Total aerobic microbial count ≤800cfu/g, Total yeasts and molds ≤80cfu/g, Escherichia coli should not be detected (for oral use) or Total aerobic microbial count ≤80cfu/g, Total yeasts and molds ≤10cfu/g, Escherichia coli should not be detected (for injection) |
含量 Content | 含NaHCO3应为99.0~101.0% NaHCO3 content should be 99.0~101.0% | 含NaHCO3应为99.5~100.5%(供注射、血液透析用)或不得少于99.0%(供口服用) NaHCO3 content should be 99.5~100.5% (for injection, hemodialysis) or no more than 99.0% (for oral use) |










